具有NASICON结构的Na3V2(PO4)3 (NVP)离子电导率高、体积变形小、理论比能量高(400 Wh/kg)和
电化学稳定性高,是
钠离子电池正极的候选材料之一[1~3]
但是,NVP较低的本征电子电导率使其倍率性能和循环性能不高
降低颗粒尺寸、掺杂和碳包覆,可提高其导电性[4]
为了制备小尺寸的NVP颗粒,可用溶胶–凝胶法[5]、水热法[6]、喷雾干燥法[7,8]和溶液燃烧合成(Solution combustion synthesis,SCS)[9~12]等液相法,调节工艺参数可控制其形貌和尺寸
SCS是一种简单、快速、高效的液相制备方法
Wang等[9]用SCS合成的碳包覆NVP钠离子电池
正极材料,在0.1 C下的初始充放电比容量分别为111和101 mAh·g–1,循环50圈后的容量保持率为95%
但是,SCS的反应过程难以控制且产物易发生团聚
超声技术是一种能强化化学反应和化工过程的物理手段,可用于辅助制备
纳米材料[13~15]
超声的力学效应和空化效应不仅能促进反应的进行,还能有效阻止颗粒的团聚和长大[16]
将超声技术引入SCS过程,可合成碳包覆NVP[17]
超声的引入使用传统SCS合成的块状团聚体转变成富含孔洞的3D网络结构,还能降低NVP的晶粒尺寸(<20 nm)
这种材料在0.1 C下的比容量可提高到117 mAh·g–1,在2 C下的比容量为85 mAh·g–1,在0.2 C下循环120次后保持初始容量的94%
但是在SCS过程中生成的碳为无定形碳,不能将NVP颗粒完全包覆也不能在NVP颗粒之间建立连续的三维导电网络结构
石墨烯是一种比表面积较大的纳米碳材料,具有良好的导电性和优良的机械性能,是很有前途的导电碳材料[18~22]
将大表面的石墨烯掺入NVP中可将NVP颗粒联结起来构建连续的电子三维通道从而提高NVP的导电性
为了进一步提高超声辅助溶液燃烧合成的NVP的导电性从而提高其倍率性能和循环性能,本文用超声辅助溶液燃烧法合成硬碳和石墨烯双层碳包覆的NVP
复合材料,研究添加石墨烯对其组织结构和电化学性能的影响
1 实验方法1.1 双层碳包覆NVP的制备
用Hummers法[23,24]制备氧化石墨烯(GO)纳米片
双层碳包覆NVP的制备:将2.55 g硝酸钠(NaNO3)、3.45 g的磷酸二氢氨(NH4H2PO4)、2.34 g偏矾酸铵(NH4VO3)和8.45 g柠檬酸(C6H8O7)溶解在去离子水,得到100 mL澄清的前驱体溶液
在前驱体溶液中加入GO(添加量分别为0.0%、2.5%、5.0%)并超声2 h,然后将装有前驱体溶液的坩埚放到温度为500℃的电阻炉内,将超声杆的顶端浸入前驱体溶液中并开启超声
在超声场和温度场的作用下溶液沸腾、蒸发、浓缩和燃烧,得到蓬松的前驱体粉末
将前驱体粉末在Ar+H2(H2的体积比为5%)气氛中热处理,在800℃保温4 h后得到碳包覆NVP复合材料
1.2 样品性能的表征
用X' Pert PRO型X射线衍射仪表征材料的物相,Cu靶,Kα 射线源(λ=0.15418 nm),电压和电流分别为40 kV和40 mA,扫描速度为10 (°)/min,扫描范围2θ为10°~60°
用LabRAM HR800型拉曼光谱分析仪测试Raman谱(使用波长为532 nm的激光,波数范围为1000~2000 cm–1)
用Nova NanoSEM 450扫描电镜和Tecnai G2 F30透射电子显微镜观察样品的微观形貌、颗粒尺寸和碳包覆
用3H-2000PM1型比表面积分析仪测试粉末的N2吸附-脱附性能
按质量比8:1:1的比例将活性物质碳包覆Na3V2(PO4)3、科琴黑和聚偏氟乙烯(PVDF)混合后研磨使之均匀,加入适量的N-甲基吡咯烷酮(NMP)继续研磨后得到混合均匀的浆料
将浆料均匀地涂覆在铝箔表面在80℃干燥12 h,然后用压片机压实并切成直径为8 mm的小圆片作为正极
在充满Ar气的手套箱内组装CR2032型扣式电池(水和氧的含量均小于1×10–6,体积分数),其中对电极和参考电极为金属钠片,隔膜为玻璃纤维,电解液为含有1 mol·L–1 NaClO4的EC+PC溶液(其中EC、PC的体积比为1:1)
在室温下(25℃)测试电池的电化学性能,包括电化学阻抗(使用CHI600D型电化学工作站)、恒流充放电性能(使用CT2001A型Land电池测试系统)
对于Na3V2(PO4)3/C/GO电极,充放电电压区间为2.3~3.9V,以1C电流密度测试充放电循环性能,测试周期为300圈
在1、2、5、10 C的电流密度(1C=117 mAh·g–1)下测试倍率性能,在每种电流密度下循环5圈
2 结果和讨论2.1 NVP的组织结构
图1给出了GO添加量不同的碳包覆NVP的XRD谱
可以看出,GO添加量不同的样品其衍射峰位和相对强度相似,衍射峰与NVP的JCPDS No: 53-0018相符合,表明三种样品均为结晶性良好的NVP
在XRD谱中未观察到GO的衍射峰,表明GO以纳米片无序堆叠的形式存在[25]
可用Scherrer公式
D=Kλ/Bcosθ
计算粉末的平均晶粒尺寸,其中D为样品的晶粒垂直于晶面方向的平均厚度(nm),K为Scherrer常数(0.89),B为试样宽化(实测样品最强衍射峰对应的半峰宽扣除仪器固有宽化),θ为最强衍射峰对应的衍射角,λ为X-射线波长0.15418 nm
以(116)晶面计算的结果表明,添加GO含量分别为0.0%、2.5%和5.0%的NVP其晶粒尺寸分别为16.6 nm、16.9 nm、17.0 nm,相差不大
图1
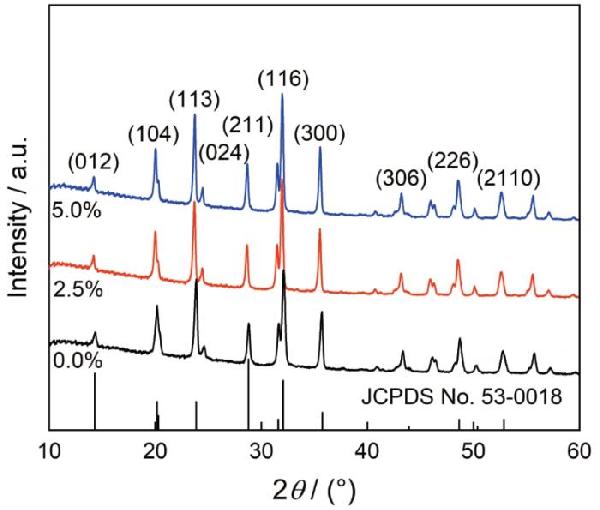
图1碳包覆NVP的XRD谱
Fig.1XRD patterns of carbon-coated NVP
将前驱体溶液加热至沸点(500℃),沸腾时溶剂挥发,溶液浓缩,浓缩到一定程度溶质开始分解
达到点燃时溶液燃烧发生氧化还原反应,其中硝酸盐为氧化剂,柠檬酸为还原剂
三种实验现象相似
结合XRD谱的结果表明,GO的加入未改变燃烧合成反应
图2给出了自制GO和GO添加量不同的NVP的拉曼谱
可以看出,在三个NVP样品的谱里都出现了碳的两个特征峰,分别位于1300 cm–1(D峰)和1600 cm–1(G峰)附近,与无定形碳和石墨化碳对应
未加入GO的样品其G峰并不明显,表明在SCS过程中生成的碳主要为无定形态
随着GO添加量的增加G峰变得显著,表明出现了石墨烯
根据峰面积计算出比值ID/IG,得到GO含量为0.0%、2.5%和5.0%三个样品的ID/IG值分别为2.1、1.5和1.3,表明C的有序度提高了
图2

图2碳包覆NVP的拉曼谱
Fig.2Raman spectra of carbon-coated NVP
图3给出了自制石墨烯纳米片的TEM照片
可见纳米片的尺寸约为几个微米到十几个微米,厚度约为几个纳米
图4给出了GO添加量不同的NVP的SEM照片
可以看出,三种产物均由团聚颗粒组成,每个团聚颗粒都具有均匀的三维孔结构,孔径为1~5 μm
但是从图4右上角的插图可见,NVP团聚颗粒的次级结构不同
不添加GO的NVP团聚颗粒具有珊瑚结构,因为次级颗粒相互联结形成珊瑚结构;GO添加量为2.5%时珊瑚结构变少,而分散的颗粒增多;随着GO的添加量增加到5.0%珊瑚结构基本消失,团聚颗粒由分散的小颗粒和一些片状颗粒组成,分别是NVP颗粒和石墨烯
图3

图3石墨烯纳米片的TEM照片
Fig.3TEM image of graphene nanosheets
图4

图4GO添加量不同的碳包覆NVP的SEM照片
Fig.4SEM images of carbon-coated NVP (a) 0.0%; (b) 2.5%; (c) 5.0%
用HRTEM进一步观察碳包覆NVP的显微结构,结果在图5中给出
从图5a可见,不添加GO时NVP颗粒相互联结,宏观上表现出珊瑚结构
图5d中的高倍TEM照片清楚地显示出晶格条纹,晶格间距为0.217 nm,对应NVP的(306)晶面
同时,在晶粒表面还观察到了厚度为1~2 nm的无定形碳薄层
其原因是,在溶液燃烧过程中以C6H8O7作为燃烧反应的还原剂和各金属离子的络合剂,在燃烧过程中富余的C6H8O7分解后在NVP表面形成了C包覆层
由图5b可见,GO添加量为2.5%时大部分NVP颗粒不再彼此联结
从图5e中的高倍TEM照片可观察到晶粒表面包覆着双层碳,内层是厚度为1~2 nm的无定形碳,外层是厚度为2~3 nm的GO
由图5c可见,随着GO的添加量增加到5.0% NVP颗粒趋近于球形,平均颗粒尺寸约为25 nm,分散在层片状GO上
图5f表明,NVP晶粒表面仍然具有双层碳结构,内层是厚度为1~2 nm的无定形碳,外层GO的厚度为4~5 nm
由此可见,GO含量的提高使GO层的厚度增大
图5
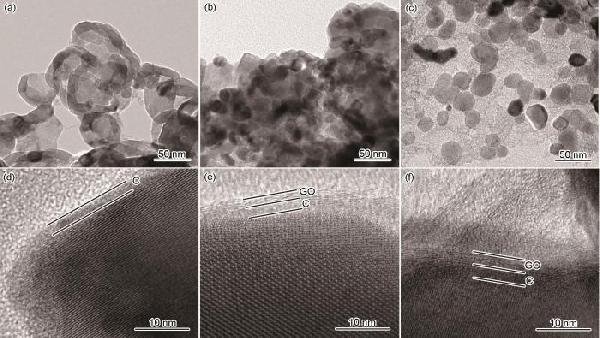
图5GO添加量不同的NVP的HRTEM图
Fig.5HRTEM images of NVP with different GO contents (a, d) 0.0%; (b, e) 2.5%; (c, f) 5.0%
在超声辅助溶液燃烧过程中,前驱体溶液内产生了大量的、均匀分布的空化泡
超声引起的力学效应,有助于小气泡在流体介质中的均匀分散
因此,燃烧后合成的产物具有均匀的蜂窝结构,燃烧产物热处理后这些蜂窝结构形成均匀的三维网络结构[17]
另外,石墨烯具有大的比表面积,为NVP晶核的形成提供大量的形核点并抑制NVP晶粒的长大
因此,石墨烯的加入可阻止颗粒的团聚,使颗粒均匀分布
图6给出了GO添加量不同的碳包覆NVP的N2吸-脱附等温曲线及孔径分布
由图6可见,随着GO添加量的增加产物的比表面积增大,分别为3.05、5.49、8.74 m2·g–1
孔径分布表明,GO添加量增加至5%,产物中尺寸为~2nm的微孔和~30nm的介孔的体积明显增多
其原因,一方面,超声产生的空化效应有助于介孔和微孔的形成;另一方面,石墨烯的比表面积大且易卷曲,也有助于增大产物表面积和形成更多的介孔和微孔
图6

图6GO添加量不同的NVP的N2吸/脱附等温曲线(内嵌为孔径分布图)
Fig.6Nitrogen adsorption/desorption isotherms of NVP with different GO contents. Inset: the pore-size distribution plot calculated by the BJH method in the adsorption branch isotherm
2.2 NVP的电化学性能
图7给出了不同GO添加量的NVP的电化学阻抗奈奎斯特图
每个奈奎斯特图都由一个位于中高频区的半圆和一条位于低频区的斜线组成
半圆表示电解质和电极之间的电荷转移电阻(Rct),斜线表示钠离子扩散引起的Warburg电阻
根据图7中的插图的等效电路可以得到GO含量为0.0%、2.5%和5.0%的NVP其Rct分别为848、485和201 Ω·cm2
GO为5.0%的NVP阻抗最低,因为足够的GO使分散的NVP颗粒彼此连接,为电子传输构建起三维通道,有助于提高NVP的动力学特性
图7
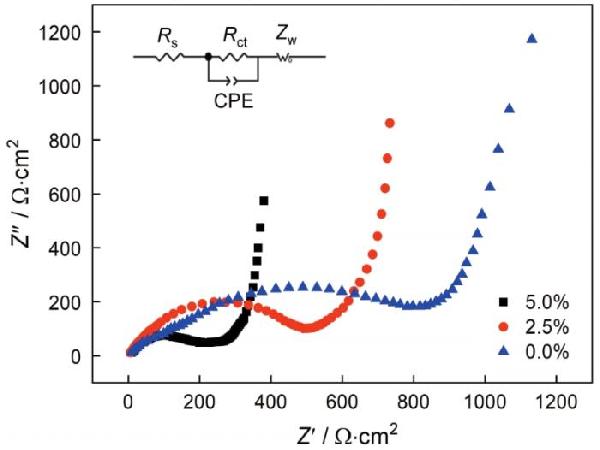
图7GO添加量不同的NVP的电化学阻抗奈奎斯特图
Fig.7Electrochemical impedance spectroscopy with different GO contents
图8a给出了GO添加量不同的NVP在1 C电流密度下的充放电曲线,电压窗口为2.3~3.9 V
可以看出,三种电极材料的充/放电曲线在3.4V左右均有一个平台,对应V3+/4+的转变
图8b给出了碳包覆NVP充放电的循环性能图(1C的电流密度)
GO含量为0.0%的NVP样品其初始比容量为99 mAh·g–1,循环120圈后比容量衰减到23 mAh·g–1
GO含量为2.5%的NVP样品其初始比容量为104 mAh·g–1,循环240圈后比容量衰减到70 mAh·g–1,容量保持率为67%
GO含量为5.0%的样品其初始比容量为117 mAh·g–1,与NVP的理论比容量接近
循环300圈后,比容量仍高达93 mAh·g–1,容量保持率为79%
图8c给出了GO含量为5.0%的NVP的库伦效率曲线,可见其首效为83%,5次循环后库伦效率接近100%
这表明,NVP的结构稳定,硬碳和石墨烯所构成的三维导电网络有利于NVP的氧化还原反应,从而增强其电化学循环稳定性
图8d给出了石墨烯添加量不同的NVP的倍率性能
在各个倍率下,GO添加量为5.0%的NVP的比容量最优
GO含量为5.0%的样品在不同倍率下的首次放电比容量分别为117 mAh·g–1(1 C)、112 mAh·g–1(2 C)、108 mAh·g–1(5 C)、100 mAh·g–1(10 C),相应倍率下的比容量保持率分别为98%、95%、88%
这些结果表明,该样品作为正极材料有很好的倍率性能,特别是在大倍率下电池依然具有较高的比容量
图8

图8GO添加量不同的NVP的电化学性能
Fig.8Electrochemical performance of NVP with different GO contents (a) charge-discharge profiles at 1 C; (b) cycling performance at 1 C; (c) coulombic efficiency of NVP (GO: 5.0%) at 1 C; (d) rate capability at various current rates from 1 C to 10 C
比较上述的电化学性能,GO添加量为5.0%的NVP具有良好的电化学性能
其原因是:(1)超声辅助SCS过程及石墨烯的协同作用有助于高分散NVP纳米颗粒的生成,纳米尺寸的NVP颗粒有较小的Na+的迁移路径,有利于Na+的高速脱嵌
(2)在SCS过程中生成的无定形碳包覆在NVP颗粒表面,促进了电子转移并能阻止NVP和电解液直接接触,有利于提高界面稳定性
(3)大片石墨烯连接纳米NVP颗粒,为电子传输构建起三维通道
因此,无定形碳和石墨烯的共同作用使NVP的导电性提高
3 结论
采用超声辅助溶液燃烧合成技术可制备双层碳包覆NVP复合材料,其颗粒表面由内向外包覆着无定形碳和石墨烯
添加石墨烯有助于形成丰富的多级孔结构,并提高NVP颗粒的分散性和降低其尺寸
石墨烯添加量为5.0%的GO和硬碳为NVP颗粒的电子传输构建起了三维通道,使材料的电化学性能提高
在1 C倍率下充放电,NVP的初始比容量有117 mAh·g-1,循环300圈后容量保持率为79%,在10 C倍率下放电比容量高达100 mAh·g-1
参考文献
View Option 原文顺序文献年度倒序文中引用次数倒序被引期刊影响因子
[1]
Saravanan K, Mason C W, Rudola A, et al.
The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries
[J]. Adv. Energy Mater., 2013, 3(4): 444
DOIURL [本文引用: 1]
[2]
Song W X, Ji X B, Wu Z P, et al.
First exploration of Na-ion migration pathways in the NASICON structure Na3V2(PO4)3
[J]. J. Mater. Chem., 2014, 2A(15) : 5358
[3]
Jian Z L, Han W Z, Lu X, et al.
Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries
[J]. Adv. Energy Mater., 2013, 3(2): 156
DOIURL [本文引用: 1]
[4]
Zhang B W, Ma K X, Lv X, et al.
Recent advances of NASICON-Na3V2(PO4)3 as cathode for sodium-ion batteries: synthesis, modifications, and perspectives
[J]. J. Alloys Compd., 2021, 867: 159060
DOIURL [本文引用: 1]
[5]
Wang E H, Chen M Z, Liu X H, et al.
Organic cross-linker enabling a 3D porous skeleton-supported Na3V2(PO4)3/carbon composite for high power sodium-ion battery cathode
[J]. Small, 2018, 3: 1800169
[本文引用: 1]
[6]
Cao X X, Pan A Q, Yin B, et al.
Nanoflake-constructed Porous Na3V2(PO4)3/C hierarchical microspheres as a bicontinuous cathode for sodium-ion batteries applications
[J]. Nano Energy, 2019, 60: 312
DOIURL [本文引用: 1]
[7]
Zhang J X, Fang Y J, Xiao L F, et al.
Graphene-scaffolded Na3V2(PO4)3 microsphere cathode with high rate capability and cycling stability for sodium ion batteries
[J]. ACS Appl. Mater. Interfaces, 2017, 9: 7177
DOIURL [本文引用: 1]
[8]
Xu J Y, Gu E L, Zhang Z Z, et al.
Fabrication of porous Na3V2(PO4)3/reduced graphene oxide hollow spheres with enhanced sodium storage performance
[J]. J. Colloid Interface Sci., 2020, 567: 84
DOIURL [本文引用: 1]
[9]
Wang H, Jiang D L, Zhang Y, et al.
Self-combustion synthesis of Na3V2(PO4)3 nanoparticles coated with carbon shell as cathode materials for sodium-ion batteries
[J]. Electrochim. Acta, 2015, 155: 23
DOIURL [本文引用: 2]
[10]
V?li R, Aruv?li J, H?rmas M, et al.
Glycine-nitrate process for synthesis of Na3V2(PO4)3 cathode material and optimization of glucose-derived hard carbon anode material for characterization in full cells
[J]. Batteries, 2019, 5(3): 56
DOIURL
[11]
Salehi A H, Masoudpanah S M, Hasheminiasari M, et al.
A solution synthesis of Na3V2(PO4)3 cathode for sodium storage by using CTAB additive
[J]. Solid State Ion., 2020, 347: 115269
DOIURL
[12]
Li N L, Tong Y W, Yi D W, et al.
3D interconnected porous carbon coated Na3V2(PO4)3/C composite cathode materials for sodium-ion batteries
[J]. Ceram. Int., 2020, 46(17): 27493
DOIURL [本文引用: 1]
[13]
Sancheti S V, Gogate P R.
A review of engineering aspects of intensification of chemical synthesis using ultrasound
[J]. Ultrason. Sonochem., 2017, 36: 527
DOIPMID [本文引用: 1] " />
The chemical and physical effects of ultrasound with a frequency above 16kHz, higher than the audible frequency of the human ear, have proven to be a useful tool for variety of systems ranging from the application of ultrasound in environmental remediation to the cooperation of ultrasound waves with chemical processing regarding as sonochemistry. Ultrasound opened up new advances in textile wet processing including desizing, scouring, bleaching, dyeing, printing and finishing and also nanoprocessing including nanopretreatment, nanodyeing, nanoprinting and nanofinishing. Use of ultrasound appears to be a promising alternative technique to reduce energy, chemicals and time involved in various operations. Over the past years there has been an enormous effort on using sonochemistry for the synthesis of nanomaterials on various textile materials. In situ sonosynthesis of nanoparticles and nanocomposites on different textiles is a pioneering approach driving future investigations. With such wide range of applications and vast ever increasing publications, the objective of this paper is presenting a comprehensive review on ultrasound application in textile from early time to now by the main emphasis on the sonosynthesis of nanomaterials outlining directions toward future research. Copyright ? 2014 Elsevier B.V. All rights reserved.
[15]
Que A Z, Zhu T Y, Zheng Y Y.
Preparation of hollow magnetic graphene oxide and its adsorption performance for methylene blue
[J]. Chin. J. Mater. Res., 2021, 35(7): 517
DOI [本文引用: 1] " />
用共沉淀法将Fe<sub>3</sub>O<sub>4</sub>沉淀在PS微球上并用甲苯去除PS制备出Fe<sub>3</sub>O<sub>4</sub>@PS,再用超声将用Hummers法制备的氧化石墨烯包裹在Fe<sub>3</sub>O<sub>4</sub>@PS表面制备出中空磁性氧化石墨烯,研究了这种复合材料对模拟亚甲基蓝废水的吸附
结果表明:在55℃,用中空磁性氧化石墨烯对亚甲基蓝染料吸附60 min达到平衡,最大吸附量为349.85 mg·g<sup>-1</sup>
吸附剂循环8次,吸附效率仍高于80%
用准二级动力学模型可很好地拟合中空磁性氧化石墨烯对亚甲基蓝的吸附
结果表明,吸附速率对亚甲基蓝染料的初始浓度较为敏感,主要为化学吸附
吸附过程符合Langmuir等温吸附模型,说明这种吸附为单层表面吸附
[16]
Suslick K S.
Sonochemistry
[J]. Science, 1990, 247(4949): 1439
DOIPMID [本文引用: 1] class="outline_tb" " />
Cavitation generated using ultrasound can enhance the rates of several chemical reactions giving better selectivity based on the physical and chemical effects. The present review focuses on overview of the different reactions that can be intensified using ultrasound followed by the discussion on the chemical kinetics for ultrasound assisted reactions, engineering aspects related to reactor designs and effect of operating parameters on the degree of intensification obtained for chemical synthesis. The cavitational effects in terms of magnitudes of collapse temperatures and collapse pressure, number of free radicals generated and extent of turbulence are strongly dependent on the operating parameters such as ultrasonic power, frequency, duty cycle, temperature as well as physicochemical parameters of liquid medium which controls the inception of cavitation. Guidelines have been presented for the optimum selection based on the critical analysis of the existing literature so that maximum process intensification benefits can be obtained. Different reactor designs have also been analyzed with guidelines for efficient scale up of the sonochemical reactor, which would be dependent on the type of reaction, controlling mechanism of reaction, catalyst and activation energy requirements. Overall, it has been established that sonochemistry offers considerable potential for green and sustainable processing and efficient scale up procedures are required so as to harness the effects at actual commercial level.Copyright ? 2016 Elsevier B.V. All rights reserved.
[14]
Harifi T, Montazer M.
A review on textile sonoprocessing: a special focus on sonosynthesis of nanomaterials on textile substrates
[J]. Ultrason. Sonochem., 2015, 23: 1
PMID " />
The Fe3O4 coated polystyrene microsphere (PS), namely Fe3O4@PSwas firstly fabricated by co-precipitation method with FeCl2·6H2O and FeCl3 as raw material, and PS microsphere as tempelate. Then Fe3O4@PS was immersed in toluene solution for removing the PS template. Next, the hollow Fe3O4 microsphere was coated with graphene oxide sheets under sonication to produce the hollow magnetic graphene oxide (HMGO). Subsequently, the absorption performance of the HMGO for methylene blue (MB) was assessed in an artificial waste MB solution. Results verified that the adsorption process reach to equilibrium at 55℃ after 60 min. The maximum adsorption capacity of MB on HMGO is 349.85 mg·g-1. The adsorbent shows good stability and reusability, after 8 times recycling the adsorption rate is still higher than 80%. The adsorption process of MB on HMGO can be well fitted by Pseudo-second-order kinetic model and the adsorption rate is sensitive to the initial concentration. The adsorption isotherm conforms to the Langmuir isotherm model, and the adsorption process is a single-layer surface adsorption.
阙爱珍, 朱桃玉, 郑玉婴.
中空磁性氧化石墨烯的制备及其对亚甲基蓝吸附性能
[J]. 材料研究学报, 2021, 35(7): 517
![]()
用共沉淀法将Fe<sub>3</sub>O<sub>4</sub>沉淀在PS微球上并用甲苯去除PS制备出Fe<sub>3</sub>O<sub>4</sub>@PS,再用超声将用Hummers法制备的氧化石墨烯包裹在Fe<sub>3</sub>O<sub>4</sub>@PS表面制备出中空磁性氧化石墨烯,研究了这种复合材料对模拟亚甲基蓝废水的吸附
结果表明:在55℃,用中空磁性氧化石墨烯对亚甲基蓝染料吸附60 min达到平衡,最大吸附量为349.85 mg·g<sup>-1</sup>
吸附剂循环8次,吸附效率仍高于80%
用准二级动力学模型可很好地拟合中空磁性氧化石墨烯对亚甲基蓝的吸附
结果表明,吸附速率对亚甲基蓝染料的初始浓度较为敏感,主要为化学吸附
吸附过程符合Langmuir等温吸附模型,说明这种吸附为单层表面吸附
[16]
Suslick K S.
Sonochemistry
[J]. Science, 1990, 247(4949): 1439
PMID
![]()
Ultrasound causes high-energy chemistry. It does so through the process of acoustic cavitation: the formation, growth and implosive collapse of bubbles in a liquid. During cavitational collapse, intense heating of the bubbles occurs. These localized hot spots have temperatures of roughly 5000 degrees C, pressures of about 500 atmospheres, and lifetimes of a few microseconds. Shock waves from cavitation in liquid-solid slurries produce high-velocity interparticle collisions, the impact of which is sufficient to melt most metals. Applications to chemical reactions exist in both homogeneous liquids and in liquid-solid systems. Of special synthetic use is the ability of ultrasound to create clean, highly reactive surfaces on metals. Ultrasound has also found important uses for initiation or enhancement of catalytic reactions, in both homogeneous and heterogeneous cases.
[17]
Chen Q Y, Liu Q, Chu X C, et al.
Ultrasonic-assisted solution combustion synthesis of Porous Na3V2(PO4)3/C: formation mechanism and sodium storage performance
[J]. J. Nanopart. Res., 2017, 19: 146
[本文引用: 2]
[18]
Li S, Dong Y F, Xu L, et al.
Effect of carbon matrix dimensions on the electrochemical properties of Na3V2(PO4)3 nanograins for high-performance symmetric sodium-ion batteries
[J]. Adv. Mater., 2014, 26(21): 3545
[19]
Fang Y J, Xiao L F, Ai X P, et al.
Hierarchical carbon framework wrapped Na3V2(PO4)3 as a superior high-rate and extended lifespan cathode for sodium-ion batteries
[J]. Adv. Mater., 2015, 27(39): 5895
[20]
Zhu C B, Kopold P, Van Aken P A, et al.
High power-high energy sodium battery based on threefold interpenetrating network
[J]. Adv. Mater., 2016, 28(12): 2409
[21]
Xu Y N, Wei Q L, Xu C, et al.
Layer-by-layer Na3V2(PO4)3 embedded in reduced graphene oxide as superior rate and ultralong-life sodium-ion battery cathode
[J]. Adv. Energy Mater., 2016, 6(14): 1600389
[22]
Rui X H, Sun W P, Wu C, et al.
An advanced sodium-ion battery composed of carbon coated Na3V2(PO4)3 in a porous graphene network
[J]. Adv. Mater., 2015, 27(42): 6670
[23]
Hummers W S, Offeman R E.
Preparation of graphitic oxide
[J]. J. Am. Chem. Soc., 1958, 80(6): 1339
[24]
Purushothaman K K, Saravanakumar B, Babu I M, et al.
Nanostructured CuO/reduced graphene oxide composite for hybrid supercapacitors
[J]. RSC Adv., 2014, 4(45): 23485
[25]
Zhang J T, Xiong Z G, Zhao X S.
Graphene-metal-oxide composites for the degradation of dyes under visible light irradiation
[J]. J. Mater. Chem., 2011, 21(11): 3634
The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries
1
2013
声明:
“钠离子电池双层碳包覆Na3V2(PO4)3 正极材料的超声辅助溶液燃烧合成及其电化学性能” 该技术专利(论文)所有权利归属于技术(论文)所有人。仅供学习研究,如用于商业用途,请联系该技术所有人。
我是此专利(论文)的发明人(作者)

 712
编辑:中冶有色技术网
来源:罗昱,陈秋云,薛丽红,张五星,严有为
712
编辑:中冶有色技术网
来源:罗昱,陈秋云,薛丽红,张五星,严有为








 举报 0
举报 0
 收藏 0
收藏 0
 反对 0
反对 0
 点赞 0
点赞 0

